 |
| The transparent conductive film under an electron microscope: the crystals are grown widthways, close together using a "molecular lid" - giving the film optimum conductivity. @ EMPA |

The mission of the NWA is to globally promote nanotechnology solutions adoption across industries by connecting entrepreneurs with researchers, start-ups with investors, providers with potential customers, employers with job seekers, key players with one another, in an independent and mainly industry-oriented advocacy group – inclusive of business, academia, business-supporting associates, as well as affiliate government agencies and other associations.
Tuesday, April 29, 2014
Environmentally friendly production method for transparent conductive films
Monday, April 28, 2014
How to create nanowires only three atoms wide with an electron beam
 |
| Series of still scanning electron micrographs (a to d) show how the electron beam is used to create nanowires. (Junhao Lin / Vanderbilt) |
Lin’s achievement is described in an article published online on April 28 by the journal Nature Nanotechnology. According to his advisor Sokrates Pantelides, University Distinguished Professor of Physics and Engineering at Vanderbilt University, and his collaborators at ORNL, the technique represents an exciting new way to manipulate matter at the nanoscale and should give a boost to efforts to create electronic circuits out of atomic monolayers, the thinnest possible form factor for solid objects.
 |
| Graduate student Junhao Lin (Jason Richards / Oak Ridge National Laboratory) |
“Junhao took this project and really ran with it,” said Pantelides.
Lin made the tiny wires from a special family of semiconducting materials that naturally form monolayers. These materials, called transition-metal dichalcogenides (TMDCs), are made by combining the metals molybdenum or tungsten with either sulfur or selenium. The best-known member of the family is molybdenum disulfide, a common mineral that is used as a solid lubricant.
Atomic monolayers are the object of considerable scientific interest these days because they tend to have a number of remarkable qualities, such as exceptional strength and flexibility, transparency and high electron mobility. This interest was sparked in 2004 by the discovery of an easy way to create graphene, an atomic-scale honeycomb lattice of carbon atoms that has exhibited a number of record-breaking properties, including strength, electricity and heat conduction. Despite graphene’s superlative properties, experts have had trouble converting them into useful devices, a process materials scientists call functionalization. So researchers have turned to other monolayer materials like the TMDCs.
Other research groups have already created functioning transistors and flash memory gates out of TMDC materials. So the discovery of how to make wires provides the means for interconnecting these basic elements. Next to the transistors, wiring is one of the most important parts of an integrated circuit. Although today’s integrated circuits (chips) are the size of a thumbnail, they contain more than 20 miles of copper wiring.
Because this technique uses electron irradiation, it can in principle be applicable to any kind of electron-based instrument, such as electron-beam lithography.”“This will likely stimulate a huge research interest in monolayer circuit design,” Lin said. “Because this technique uses electron irradiation, it can in principle be applicable to any kind of electron-based instrument, such as electron-beam lithography.”
One of the intriguing properties of monolayer circuitry is its toughness and flexibility. It is too early to predict what kinds of applications it will produce, but “If you let your imagination go, you can envision tablets and television displays that are as thin as a sheet of paper that you can roll up and stuff in your pocket or purse,” Pantelides commented.
In addition, Lin envisions that the new technique could make it possible to create three-dimensional circuits by stacking monolayers “like Lego blocks” and using electron beams to fabricate the wires that connect the stacked layers.
The nanowire fabrication was carried out at ORNL in the microscopy group that was headed until recently by Stephen J. Pennycook, as part of an ongoing Vanderbilt-ORNL collaboration that combines microscopy and theory to study complex materials systems. Junhao is a graduate student who pursues both theory and electron microscopy in his doctoral research. His primary microscopy mentor has been ORNL Wigner Fellow Wu Zhou.
“Junhao used a scanning transmission electron microscope (STEM) that is capable of focusing a beam of electrons down to a width of half an angstrom (about half the size of an atom) and aims this beam with exquisite precision,” Zhou said.
The collaboration included a group headed by Kazu Suenaga at the National Institute of Advanced Industrial Science and Technology in Tsukuba, Japan, where the electrical measurements that confirmed the theoretical predictions were made by post-doctoral associate Ovidiu Cretu. Other collaborators at ORNL, the University of Tennessee in Knoxville, Vanderbilt University, and Fisk University contributed to the project.
Primary funding for the research was provided by the Department of Energy’s Office of Science grant DE-FG02-09ER46554 and by the ORNL Wigner Fellowship. The work was carried at the ORNL Center for Nanophase Materials Science user facility. Computations were done at the National Energy Research Scientific Computer Center.
https://www.youtube.com/watch?v=Yz6wPhANryA
Source: http://news.vanderbilt.edu/2014/04/nanowires/
Resolving the structure of a single biological molecule
 |
| An image of the DNA double helix structure taken with the AFM, with the Watson-Crick DNA structure overlaid (purple and blue). |
Researchers at the London Centre for Nanotechnology have determined the structure of DNA from measurements on a single molecule, and found that this structure is not as regular as one might think, reports the journal Small.
Our life depends on molecular machinery that is continuously at work in our bodies. The structure of these nanometre-scale machines is thus at the heart of our understanding of health and disease. This is very apparent in the case of the Watson-Crick DNA double-helix structure, which has been the key to understanding how genetic information is stored and passed on.
Watson and Crick’s discovery was based on diffraction of X-rays by millions of ordered and aligned DNA molecules. This method is extremely powerful and still used today – in a more evolved form – to determine the structure of biological molecules. It has the important drawbacks, however, that it only provides static, averaged pictures of molecular structures and that it relies on the accurate ordering and alignment of many molecules. This process, called crystallisation, can prove very challenging.
Building on previous work in Dr Bart Hoogenboom’s research group at the London Centre for Nanotechnology, and in collaboration with the National Physical Laboratory, first author Alice Pyne has applied “soft-touch” atomic force microscopy to large, irregularly arranged and individual DNA molecules. In this form of microscopy, a miniature probe is used to feel the surface of the molecules one by one, rather than seeing them.
To demonstrate the power of their method, Pyne, Hoogenboom and collaborators have measured the structure of a single DNA molecule, finding on average good agreement with the structure as it has been known since Watson and Crick. Strikingly, however, the single-molecule images also reveal significant variations in the depths of grooves in the double helix structure.
While the origin of the observed variations is not yet fully understood, it is known that these grooves act as keyways for proteins (molecular keys) that determine to which extent a gene is expressed in a living cell. The observation of variations in these keyways may thus help us to determine the mechanisms by which living cells promote and suppress the use of genetic information stored in their DNA.
Journal link: Single-molecule reconstruction of oligonucleotide secondary structure by atomic force microscopy, Small (2014), manuscript smll.201400265R1
Source: https://www.london-nano.com/research-and-facilities/highlight/resolving-the-structure-of-a-single-biological-molecule
Thursday, April 24, 2014
What is a Neutrino?

A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with half-integer spin. The neutrino (meaning "little neutral one" in Italian) is denoted by the Greek letter ν (nu). All evidence suggests that neutrinos have mass but the upper bounds established for their mass are tiny even by the standards of subatomic particles.
Neutrinos do not carry electric charge, which means that they are not affected by the electromagnetic forces that act on charged particles such as electrons and protons. Neutrinos are affected only by the weak sub-atomic force, of much shorter range than electromagnetism, and gravity, which is relatively weak on the subatomic scale. Therefore a typical neutrino passes through normal matter unimpeded.
Neutrinos are created as a result of certain types of radioactive decay, or nuclear reactions such as those that take place in the Sun, in nuclear reactors, or when cosmic rays hit atoms. There are three types, or "flavors", of neutrinos: electron neutrinos, muon neutrinos and tau neutrinos. Each type is associated with an antiparticle, called an "antineutrino", which also has neutral electric charge and half-integer spin. Whether or not the neutrino and its corresponding antineutrino are identical particles has not yet been resolved, even though the antineutrino has an opposite chirality to the neutrino.
Most neutrinos passing through the Earth emanate from the Sun. About 6.5×1010 solar neutrinos per second pass through every square centimeter perpendicular to the direction of the Sun in the region of the Earth.
Physicists at the CERN laboratory have put the final nail in the coffin for the idea that neutrinos can travel faster than the speed of light. They also confirmed that the groundbreaking results from 2011 can be blamed on faulty equipment.
[partner id="wireduk"] Back in September 2011, a team of particle physicists detected neutrinos moving faster than the speed of light as they traveled from CERN to the Gran Sasso lab. They smashed the universal speed limit by 60 nanoseconds — a result that was constant, even after 15,000 repetitions of the process.
The results seem to run counter to a century’s worth of physics and would overturn Einsten’s special theory of relativity if true. As such, CERN called for more experiments to double-check the findings.
“When an experiment finds an apparently unbelievable result, it’s normal procedure to invite broader scrutiny,” CERN research director Sergio Bertolucci said at the time. “We need to be sure that there are no other, more mundane, explanations.”
At the International Conference on Neutrino Physics and Astrophysics in Kyoto on June 8, CERN research director Sergio Bertolucci presented results on the travel time of neutrinos from CERN to the INFN Gran Sasso Laboratory, on behalf of four experiments — Borexino, Icarus, LVD and Opera.
All four experiments measured a neutrino time of flight that was below the speed of light, confirming that neutrinos respect Einstein’s cosmic speed limit. The previous anomaly was “attributed to a faulty element of the experiment’s fibreoptic timing system.”
“Although this result isn’t as exciting as some would have liked,” said Bertolucci, “it is what we all expected deep down.”
“The story captured the public imagination, and has given people the opportunity to see the scientific method in action — an unexpected result was put up for scrutiny, thoroughly investigated and resolved in part thanks to collaboration between normally competing experiments.”
In March 2012, Antonio Ereditato — spokesperson of the Opera experiment — resigned from his post. That was following revelations that the results could have been affected by an unaccounted-for error, and a follow-up test from Icarus that ran counter to Opera’s findings.
Tuesday, April 22, 2014
A New Approach to Engineering the Materials of the Future
 |
| Transmission electron microscope (TEM) images and GISAXS paEerns (insets) of two giant surfactant thin‐film samples. The TEM images show ordered nanoscale paEerns. |
Some of the most interesting and fascinating electronic devices that will someday be available to consumers, from paper-thin computers to electronic fabric, will be the result of advanced materials designed by scientists. Indeed, some remarkable discoveries have already been made. To innovate further, scientists must learn how to precisely engineer the chemical structures of materials at the nanoscale in such a way as to yield specific macroscopic properties and functions.
A research group, jointly working at theNational Synchrotron Light Source, has found a new way to do just that. They have synthesized a new class of macromolecules that organize themselves, or “self-assemble,” into various ordered structures with feature sizes smaller than 10 nanometers. Called “giant surfactants,” these large molecules mimic the structural features of small surfactants (substances that significantly lower the surface tension between two liquids, such as detergents), but have been transformed into functional molecular nanoparticles by being “clicked” with polymer chains. The resulting materials are unique because they bridge the gap between small molecule surfactants and traditional block copolymers and thus possess an interesting “duality” in their self-assembly behaviors.
“This class of materials provides a versatile platform for engineering nanostructures that have features smaller than 10 nanometers, which is a scale that is very relevant to the blueprints of nanotechnology and microelectronics,” said the study’s corresponding scientist Stephen Cheng, a researcher in the University of Akron’s College of Polymer Science and Polymer Engineering. “More broadly, we are also interested in how our results could help advance our understanding of the chemical and physical principles that underlie self-assembly.”
Surfactants play a huge role in our everyday life, although most people are unaware of them. They are present in household cleaners and soaps, adhesives, paint, ink, plastics, and many, many other products. Naturally, they are a key part of materials research.
Giant surfactants have the potential to be even more versatile than their smaller counterparts because they have the advantages of both a polymer and a surfactant. They are of particular interest to the electronics industry because they can spontaneously self-assemble into nanodomains just a few nanometers in size. This length scale must be achieved in order to allow the continual downsizing of computer chips but proven very difficult to achieve for conventional technologies. The production of nanopatterned thin films – which are the foundation of modern computer chips – could be directly affected by giant surfactants. If films can be produced with smaller nanoscale features, they could lead to denser, faster computer chips.
The group used several techniques to study different giant surfactant samples in thin-film form, as well as in bulk form and in solution. These techniques included grazing-incidence small-angle x-ray scattering (GISAXS) at NSLS beamline X9. GISAXS is suited to studying thin film samples that have ordered nanoscale features, typically between 5 and 20 nanometers, and can tell researchers about the shape, size, and orientation of these features, among other information. It is widely used to study self-assembled thin films with nanoscale features.
This research is published in the June 18, 2013 issue of the Proceedings of the National Academy of Sciences. The team, which includes scientists from the University of Akron, National Tsing Hua University (Taiwan), McMaster University (Canada), and Peking University (China), has also described this research in a pending patent application.
http://www.bnl.gov/newsroom/news.php?a=24808
Friday, April 18, 2014
Flowing salt water over graphene generates electricity
 |
| Illustration of the experimental set-up @ Institute of Nanoscience, Nanjing University of Aeronautics and Astronautics |
Graphene, the “wonder material” that has caught the interest of scientists globally, could be used to make microscopic hydroelectric systems capable of powering small electronic devices, a new study has found.
Since its discovery in 2004, many experts have said graphene could change the world. It is the strongest material known to exist, though it is only one atom thick. It is remarkably pliable, almost transparent and an excellent conductor of electricity and heat
The potential applications of graphene are many, though much of the excitement has focused on the possibility of making advanced, lightweight and superfast electronics.
In 2013, the EU made available a €1 billion grant to researchers investigating the potential uses of the environmentally friendly “wonder material”, saying it could become as important as steel or plastics. Bill Gates’s philanthropic foundation has even paid for the development of a graphene-based condom.
Now, in the findings of an unprecedented study, researchers from the Nanjing University of Aeronautics and Astronautics in China have revealed that dragging small droplets of salt water along strips of graphene generates electricity.
They found that the faster they dragged the droplet across the graphene strip, the higher the voltage they generated.
The scientists then scaled up the experiment, placing a droplet of copper chloride on a tilted graphene surface, generating a voltage of approximately 30 millivolts (mV) – a millivolt being one thousandth of a volt.
Though much more research is needed, the scientists say these nano-sized generators could power small devices. This is something far beyond current hydroelectric systems – which usually work on a much larger scale.
A separate study, published in 2013, also suggested that buildings covered in graphene paint could harness sunlight to generate solar power.
Nature Nanotechnology, 2014. DOI: 10.1038/NNANO.2014.56
Thursday, April 17, 2014
Thinnest feasible membrane produced
A new nano-membrane made out of the “super material” graphene is extremely light and breathable. Not only can this open the door to a new generation of functional waterproof clothing, but also to ultra-rapid filtration.
The membrane produced by the researchers at ETH Zurich is as thin as is technologically possible.
Researchers have produced a stable porous membrane that is thinner than a nanometre. This is a 100,000 times thinner than the diameter of a human hair. The membrane consists of two layers of the much exalted ”super material” graphene, a two-dimensional film made of carbon atoms, on which the team of researchers, led by Professor Hyung Gyu Park at the Department of Mechanical and Process Engineering at ETH Zurich, etched tiny pores of a precisely defined size.
The membrane can thus permeate tiny molecules. Larger molecules or particles, on the other hand, can pass only slowly or not at all. “With a thickness of just two carbon atoms, this is the thinnest porous membrane that is technologically possible to make,” says PhD student Jakob Buchheim, one of the two lead authors of the study, which was conducted by ETH-Zurich researchers in collaboration with scientists from Empa and a research laboratory of LG Electronics. The study has just been published in journal Science.
The ultra-thin graphene membrane may one day be used for a range of different purposes, including waterproof clothing. “Our membrane is not only very light and flexible, but it is also a thousand fold more breathable than Goretex,” says Kemal Celebi, a postdoc in Park’s laboratory and also one of the lead authors of the study. The membrane could also potentially be used to separate gaseous mixtures into their constituent parts or to filter impurities from fluids. The researchers were able to demonstrate for the first time that graphene membranes could be suitable for water filtration. The researchers also see a potential use for the membrane in devices used for the accurate measurement of gas and fluid flow rates that are crucial to unveiling the physics around mass transfer at nanoscales and separation of chemical mixtures.
Breakthrough in nanofabrication
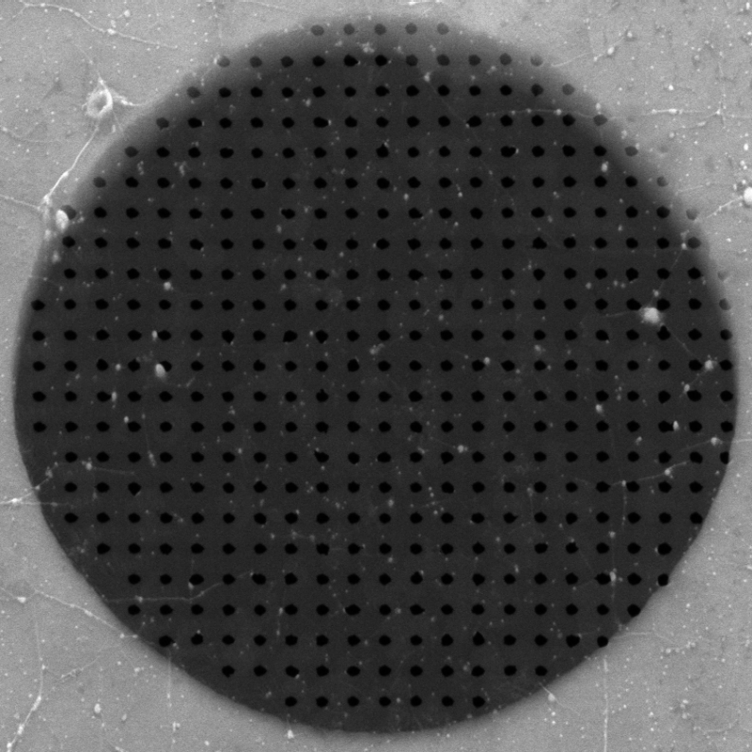 |
| Part of a graphene membrane with a multiplicity of pores (black) of precisely defined size (in this case with a diameter of 50 nanometres; photomicrograph). (Photo: Celebi K. et al. Science 2014) |
The researchers not only succeeded in producing the starting material, a double-layer graphene film with a high level of purity, but they also mastered a technique called focused ion beam milling to etch pores into the graphene film. In this process, which is also used in the production of semiconductors, a beam of helium or gallium ions is controlled with a high level of precision in order to etch away material. The researchers were able to etch pores of a specified number and size into the graphene with unprecedented precision. This process, which could easily take days to complete, took only a few hours in the current work. “This is a breakthrough that enables the nanofabrication of the porous graphene membranes,” explains Ivan Shorubalko, a scientist at Empa that also contributed to the study.
In order to achieve this level of precision, the researchers had to work with double-layer graphene. “It wouldn’t have been possible for this method to create such a membrane with only one layer because graphene in practice isn't perfect,” says Park. The material can exhibit certain irregularities in the honeycomb structure of the carbon atoms. Now and again, individual atoms are missing from the structure, which not only impairs the stability of the material but also makes it impossible to etch a high-precision pore onto such a defect. The researchers solved this problem by laying two graphene layers on top of each other. The probability of two defects settling directly above one another is extremely low, explains Park.
Fastest possible filtration
A key advantage of the tiny dimensions is that the thinner a membrane, the lower its permeation resistance. The lower the resistance, the higher the energy-efficiency of the filtration process. “With such atomically thin membranes we can reach maximal permeation for a membrane of a given pore size and we believe that they allow the fastest feasible rate of permeation,” says Celebi. However, before these applications are ready for use on an industrial scale or for the production of functional waterproof clothing, the manufacturing process needs to be further developed. To investigate the fundamental science, the researchers worked with tiny pieces of membrane with a surface area of less than one hundredth of a square millimetre. Objectives from now on will be to produce larger membrane surfaces and impose various filtering mechanisms.
https://www.ethz.ch/en/news-and-events/eth-news/news/2014/04/thinnest-feasible-membrane-produced.html
Excitons observed in action for the first time
Technique developed at MIT reveals the motion of energy-carrying quasiparticles in solid material.
A quasiparticle called an exciton — responsible for the transfer of energy within devices such as solar cells, LEDs, and semiconductor circuits — has been understood theoretically for decades. But exciton movement within materials has never been directly observed.
Now scientists at MIT and the City University of New York have achieved that feat, imaging excitons’ motions directly. This could enable research leading to significant advances in electronics, they say, as well as a better understanding of natural energy-transfer processes, such as photosynthesis.
The research is described this week in the journal Nature Communications, in a paper co-authored by MIT postdocs Gleb Akselrod and Parag Deotare, professors Vladimir Bulovic and Marc Baldo, and four others.
“This is the first direct observation of exciton diffusion processes,” Bulovic says, “showing that crystal structure can dramatically affect the diffusion process.”
“Excitons are at the heart of devices that are relevant to modern technology,” Akselrod explains: The particles determine how energy moves at the nanoscale. “The efficiency of devices such as photovoltaics and LEDs depends on how well excitons move within the material,” he adds.
An exciton, which travels through matter as though it were a particle, pairs an electron, which carries a negative charge, with a place where an electron has been removed, known as a hole. Overall, it has a neutral charge, but it can carry energy. For example, in a solar cell, an incoming photon may strike an electron, kicking it to a higher energy level. That higher energy is propagated through the material as an exciton: The particles themselves don’t move, but the boosted energy gets passed along from one to another.
While it was previously possible to determine how fast, on average, excitons could move between two points, “we really didn’t have any information about how they got there,” Akselrod says. Such information is essential to understanding which aspects of a material’s structure — for example, the degree of molecular order or disorder — might facilitate or slow that motion.
“People always assumed certain behavior of the excitons,” Deotare says. Now, using this new technique — which combines optical microscopy with the use of particular organic compounds that make the energy of excitons visible — “we can directly say what kind of behavior the excitons were moving around with.” This advance provided the researchers with the ability to observe which of two possible kinds of “hopping” motion was actually taking place.
“This allows us to see new things,” Deotare says, making it possible to demonstrate that the nanoscale structure of a material determines how quickly excitons get trapped as they move through it.
For some applications, such as LEDs, Deotare says, it is desirable to maximize this trapping, so that energy is not lost to leakage; for other uses, such as solar cells, it is essential to minimize the trapping. The new technique should allow researchers to determine which factors are most important in increasing or decreasing this trapping.
“We showed how energy flow is impeded by disorder, which is the defining characteristic of most materials for low-cost solar cells and LEDs,” Baldo says.
While these experiments were carried out using a material called tetracene — a well-studied archetype of a molecular crystal — the researchers say that the method should be applicable to almost any crystalline or thin-film material. They expect it to be widely adopted by researchers in academia and industry.
“It’s a very simple technique, once people learn about it,” Akselrod says, “and the equipment required is not that expensive.”
Exciton diffusion is also a basic mechanism underlying photosynthesis: Plants absorb energy from photons, and this energy is transferred by excitons to areas where it can be stored in chemical form for later use in supporting the plant’s metabolism. The new method might provide an additional tool for studying some aspects of this process, the team says.
David Lidzey, a professor of physics and astronomy at the University of Sheffield who was not involved in this work, calls the research “a really impressive demonstration of a direct measurement of the diffusion of triplet excitons and their eventual trapping.” He adds, “Exciton diffusion and transport are important processes in solar-cell devices, so understanding what limits these may well help the design of better materials, or the development of better ways to process materials so that energy losses during exciton migration are limited.”
The work was supported by the U.S. Department of Energy and by the National Science Foundation, and used facilities of the Eni-MIT Solar Frontiers Center.
http://newsoffice.mit.edu/2014/excitons-observed-in-action-for-first-time-0416
Monday, April 14, 2014
New Physical Phenomenon on Nanowires Seen for the First Time
 |
| False-colour scanning electron micrograph of a nanowire strain device. |
But for optical communication to happen, it is essential to convert the electrical information used in the microprocessor into light, by using light emitters. On the other end of the optical link, one needs to translate the information contained in the stream of light into electrical signals by using light detectors. Current technologies use different materials to realize these two distinct functions – silicon or germanium for light detection and materials combining elements from the III-V columns of the periodic table for light emission. However, this might be going to change soon thanks to a new discovery.
In a paper appearing today in the journal Nature Communications, scientists at IBM Research – Zurich and the Norwegian University of Science and Technology have demonstrated for the first time that both, efficient light emission and detection functionalities can be achieved in the very same nanowire material by applying mechanical strain.
Using this new physical phenomenon, scientists might be able to integrate the light emitter and the detector functions in the very same material. This would drastically reduce the complexity of future silicon nanophotonic chips.
IBM scientist Giorgio Signorello explains, "When you pull the nanowire along its length, the nanowire is in a state that we call “direct bandgap” and it can emit light very efficiently; when instead you compress the length of the wire, its electronic properties change and the material stops emitting light. We call this state “pseudo-direct”: the III-V material behaves similarly to silicon or germanium and becomes a good light detector."
IBM Fellow Heike Riel comments, “These are unique and surprising properties and they all come from the fact that the atoms are located at very special positions within the nanowire. We call this crystal structure “Wurtzite”. This structure is possible only because the nanowire dimensions are so small. You cannot achieve the same properties at dimensions visible to the eye. This is a great example of the power of nanotechnology.”
This remarkable properties might find interesting applications also outside the field of optical communication.
For the scientific background read the Nature Communications paper titled "Inducing a Direct-to-Pseudodirect Bandgap Transition in Wurtzite GaAs Nanowires with Uniaxial Stress”, doi:10.1038/ncomms4655
http://ibmresearchnews.blogspot.ch/2014/04/new-physical-phenomenon-on-nanowires.html
Saturday, April 12, 2014
Ordered arrays of nanoporous gold nanoparticles
 |
| SEM images (false color) at 25° tilt of the perfectly ordered array of the nanoporous gold nanoparticles formed from the 15 nm Au/30 nm Ag bilayers.© 2012 Wang et al; licensee Beilstein-Institut. |
The dewetting of Au/Ag bilayers on the periodically prepatterned substrates leads to the interdiffusion of Au and Ag and the formation of an array of Au–Ag alloy nanoparticles. The array of alloy nanoparticles is transformed into an array of nanoporous gold nanoparticles by a following dealloying step.
Large areas of this new type of material arrangement can be realized with this technique. In addition, this technique allows for the control of particle size, particle spacing, and ligament size (or pore size) by varying the period of the structure, total metal layer thickness, and the thickness ratio of the as-deposited bilayers.
Metallic nanoparticle arrays are attracting more and more attention due to their potential applications in plasmonics, magnetic memories, DNA detection, and catalytic nanowire growth. Nanoporous gold is very interesting for application in catalysi, for sensors, for actuators, and as electrodes for electrochemical supercapacitors. This is due to the unique structural, mechanical and chemical properties of this material. Nanoporous gold, already synthesized in the form of nanoparticles, possesses a much higher surface-to-volume ratio than bulk nanoporous gold films and gold nanoparticles. These nanoporous gold nanoparticles are expected to broaden the range of applications for both gold nanoparticles and nanoporous gold due to their two-level nanostructures (porosity of around 10 nm and particle size of a few hundreds of nanometers).
Solid-state dewetting of metal films is a simple “bottom-up” approach to fabricate nanoparticles. The dewetting of metal films is driven by reducing the surface energy of the film and the interface energy between the film and the substrate, and occurs by diffusion even well below the melting temperature of the film. In addition, alloy nanoparticles can be fabricated by exploiting the dewetting of metallic bilayers. By combining both, “top-down” approaches (such as lithography) and “bottom-up” approaches, an ordered array of metallic nanoparticles can be fabricated. The surface of the substrate is prepatterned into periodic structures by using laser interference lithography, focused ion beam (FIB), or substrate conformal imprint lithography (SCIL). During the dewetting of metal films onto prepatterned substrates, the periodic structure of the prepatterned substrates modulates the local excess chemical potential by the local curvature or by limiting the diffusion paths. This leads to the formation of 2-D nanoparticle arrays with well-defined particle size and particle spacing. Dealloying is a “bottom-up” approach to fabricate nanoporous gold by selectively removing or leaching the element Ag from the Au–Ag alloy in an Ag-corrosive environment. In this paper, perfectly ordered arrays of nanoporous gold nanoparticles are fabricated by using a combination of a “top-down” approach (SCIL) and two “bottom-up” approaches (dewetting and dealloying).
Full paper: http://www.beilstein-journals.org/bjnano/single/articleFullText.htm?publicId=2190-4286-3-74
Thursday, April 10, 2014
The Motion of the Medium Matters for Self-assembling Particles
By attaching short sequences of single-stranded DNA to nanoscale building blocks, researchers can design structures that can effectively build themselves. The building blocks that are meant to connect have complementary DNA sequences on their surfaces, ensuring only the correct pieces bind together as they jostle into one another while suspended in a test tube.
Now, a University of Pennsylvania team has made a discovery with implications for all such self-assembled structures.
Earlier work assumed that the liquid medium in which these DNA-coated pieces float could be treated as a placid vacuum, but the Penn team has shown that fluid dynamics play a crucial role in the kind and quality of the structures that can be made in this way.
As the DNA-coated pieces rearrange themselves and bind, they create slipstreams into which other pieces can flow. This phenomenon makes some patterns within the structures more likely to form than others.
The research was conducted by professors Talid Sinno and John Crocker, alongside graduate students Ian Jenkins, Marie Casey and James McGinley, all of the Department of Chemical and Biomolecular Engineering in Penn’s School of Engineering and Applied Science.
It was published in the Proceedings of the National Academy of Sciences.
The Penn team’s discovery started with an unusual observation about one of their previous studies, which dealt with a reconfigurable crystalline structure the team had made using DNA-coated plastic spheres, each 400 nanometers wide. These structures initially assemble into floppy crystals with square-shaped patterns, but, in a process similar to heat-treating steel, their patterns can be coaxed into more stable, triangular configurations.
Surprisingly, the structures they were making in the lab were better than the ones their computer simulations predicted would result. The simulated crystals were full of defects, places where the crystalline pattern of the spheres was disrupted, but the experimentally grown crystals were all perfectly aligned.
While these perfect crystals were a positive sign that the technique could be scaled up to build different kinds of structures, the fact that their simulations were evidently flawed indicated a major hurdle.
“What you see in an experiment,” Sinno said, “is usually a dirtier version of what you see in simulation. We need to understand why these simulation tools aren’t working if we’re going to build useful things with this technology, and this result was evidence that we don’t fully understand this system yet. It’s not just a simulation detail that was missing; there’s a fundamental physical mechanism that we’re not including.”
By process of elimination, the missing physical mechanism turned out to be hydrodynamic effects, essentially, the interplay between the particles and the fluid in which they are suspended while growing. The simulation of a system’s hydrodynamics is critical when the fluid is flowing, such as how rocks are shaped by a rushing river, but has been considered irrelevant when the fluid is still, as it was in the researchers’ experiments. While the particles’ jostling perturbs the medium, the system remains in equilibrium, suggesting the overall effect is negligible.
“The conventional wisdom,” Crocker said, “was that you don't need to consider hydrodynamic effects in these systems. Adding them to simulations is computationally expensive, and there are various kinds of proofs that these effects don’t change the energy of the system. From there you can make a leap to saying, ‘I don’t need to worry about them at all.’”
Particle systems like ones made by these self-assembling DNA-coated spheres typically rearrange themselves until they reach the lowest energy state. An unusual feature of the researchers’ system is that there are thousands of final configurations — most containing defects — that are just as energetically favorable as the perfect one they produced in the experiment.
“It’s like you’re in a room with a thousand doors,” Crocker said. “Each of those doors takes you to a different structure, only one of which is the copper-gold pattern crystal we actually get. Without the hydrodynamics, the simulation is equally likely to send you through any one of those doors.”
The researchers’ breakthrough came when they realized that while hydrodynamic effects would not make any one final configuration more energy-favorable than another, the different ways particles would need to rearrange themselves to get to those states were not all equally easy. Critically, it is easier for a particle to make a certain rearrangement if it’s following in the wake of another particle making the same moves.
“It’s like slipstreaming,” Crocker said. “The way the particles move together, it’s like they’re a school of fish.”
“How you go determines what you get,” Sinno said. “There are certain paths that have a lot more slipstreaming than others, and the paths that have a lot correspond to the final configurations we observed in the experiment.”
The researchers believe that this finding will lay the foundation for future work with these DNA-coated building blocks, but the principle discovered in their study will likely hold up in other situations where microscopic particles are suspended in a liquid medium.
“If slipstreaming is important here, it’s likely to be important in other particle assemblies,” Sinno said. It’s not just about these DNA-linked particles; it’s about any system where you have particles at this size scale. To really understand what you get, you need to include the hydrodynamics.”
http://www.upenn.edu/pennnews/news/motion-medium-matters-self-assembling-particles-penn-research-shows
Wednesday, April 9, 2014
UC San Diego Researchers Develop Bacterial ‘FM Radio’
Programming living cells offers the prospect of harnessing sophisticated biological machinery for transformative applications in energy, agriculture, water remediation and medicine. Inspired by engineering, researchers in the emerging field of synthetic biology have designed a tool box of small genetic components that act as intracellular switches, logic gates, counters and oscillators.
But scientists have found it difficult to wire the components together to form larger circuits that can function as “genetic programs.” One of the biggest obstacles? Dealing with a small number of available wires.
A team of biologists and engineers at UC San Diego has taken a large step toward overcoming this obstacle. Their advance, detailed in a paper which appears in this week’s advance online publication of the journal Nature, describes their development of a rapid and tunable post-translational coupling for genetic circuits. This advance builds on their development of “biopixel” sensor arrays reported in Nature by the same group of scientists two years ago.
The problem the researchers solved arises from the noisy cellular environment that tends to lead to highly variable circuit performance. The components of a cell are intermixed, crowded and constantly bumping into each other. This makes it difficult to reuse parts in different parts of a program, limiting the total number of available parts and wires. These difficulties hindered the creation of genetic programs that can read the cellular environment and react with the execution of a sequence of instructions.
The team’s breakthrough involves a form of “frequency multiplexing” inspired by FM radio.
“This circuit lets us encode multiple independent environmental inputs into a single time series,” said Arthur Prindle, a bioengineering graduate student at UC San Diego and the first author of the study. “Multiple pieces of information are transferred using the same part. It works by using distinct frequencies to transmit different signals on a common channel.”
The key that enabled this breakthrough is the use of frequency, rather than amplitude, to convey information. “Combining two biological signals using amplitude is difficult because measurements of amplitude involve fluorescence and are usually relative. It’s not easy to separate out the contribution of each signal,” said Prindle. “When we use frequency, these relative measurements are made with respect to time, and can be readily extracted by measuring the time between peaks using any one of several analytical methods.”
While their application may be inspired by electronics, the UC San Diego scientists caution in their paper against what they see as increasing “metaphorization” of engineering biology.
“We explicitly make the point that since biology is often too intertwined to engineer in the way we are accustomed in electronics, we must deal directly with bidirectional coupling and quantitatively understand its effects using computational models,” explained Prindle. “It’s important to find the right dose of inspiration from engineering concepts while making sure you aren’t being too reliant on your engineering metaphors.”

Microfluidic device containing an array of "biopixels" (square traps) in which bacteria grow. Credit: Arthur Prindle, UC San Diego
Enabling this breakthrough is the development of an intracellular wiring mechanism that enables rapid transmission of protein signals between the individual modules. The new wiring mechanism was inspired by a previous study in the lab on the bacterial stress response. It reduces the time lags that develop as a consequence of using proteins to activate or repress genes.
“The new coupling method is capable of reducing the signaling time delay between individual genetic circuits by more than an order of magnitude,” said Jeff Hasty, a professor of biology and bioengineering at UC San Diego who headed the team of researchers and co-directs the university’s BioCircuits Institute. “The state of the art has been about 20 to 40 minutes, but we can now do it in less than one minute.”
“This study is an outstanding example of the power of interdisciplinary systems biology approaches, which treat our cells like integrated pathways and networks instead of a collection of individual components,” said Sarah Dunsmore, a program manager at the National Institute of General Medical Sciences, which finances a National Center for Systems Biology at UC San Diego that supported the research. “By combining the complexity of naturally occurring biological processes with engineering principles, Dr. Hasty and colleagues have produced a model that will provide the basis for creating genetic circuits that can be used to study human health and disease.”
“What’s really exciting about this coupling method is the particular way we did it,” said Prindle. “Rather than trying to build from scratch, we made use of the enzyme machinery that the cell uses for rapid and precise signaling during times of stress. This is an appealing strategy because it lets us take advantage of the advanced machinery that nature has already evolved.”
Hasty credited Prindle for coming up with the idea for the study and carrying it through. “Beyond his modeling and bench skills, I’ve been extremely impressed by Arthur’s ingenuity and drive,” said Hasty. “This project arose from his creativity at the outset and he had the raw energy and excitement to carry it to the end.”
The scientists received funding for their development from the National Science Foundation (MCB-1121748) and by the San Diego Center for Systems Biology (NIH Grant P50 GM085764).
http://ucsdnews.ucsd.edu/pressrelease/uc_san_diego_researchers_develop_bacterial_fm_radio#When:17:00:00Z
Tuesday, April 8, 2014
Nanodot bio-organic nanochrystals will charge you phone in 30 seconds flat… in 2016

An Israeli technology company is working on a technology that could transform the semiconductor and energy-storage business. If you transform those industries, you transform modern life.
Store Dot, based in Ramat Gan, just to the west of Tel Aviv, is creating biological semiconductors that can, among other things, store a charge, emit visible light and be used to produce high-capacity, or quick-charging, batteries.
“If everything works, and we have a lot of evidence that it will do, we have a revolution in many devices—memory, batteries, the display, image sensors,” said Doron Myersdorf, chief executive of Store Dot.
The semiconductors are known as quantum dots and are made from naturally occurring organic compounds called peptides, short chains of amino acids, the building blocks of proteins. According to Gil Rosenman, chief scientist of Store Dot and holder of the Henry and Dinah Krongold Chair of Microelectronics at Tel Aviv University, when the company manipulates their chemistry, these peptides can be made to self-assemble into quantum dots—molecular-size materials that have remarkable properties.
“We take these peptides, manipulate them and manage the self-assembly process that usually takes place in nature,” said Mr. Myersdorf. “Only two molecules of peptide attach to each other, and they create a very little structure, two nanometers in size. It has very interesting properties—some are optical, some are related to charge, and others piezoelectric,” meaning they generate charge under mechanical strain.
To get some idea of the scale, the diameter of a human immunodeficiency virus is about 60 times as large.
According to Mr. Myersdorf, these peptide-based quantum dots are crystalline in nature. “That is important,” he said. “It means they are stable. They can also hold a charge. That means we can actually create a memory.”
Quantum dots are not new, but typically they have been made using inorganic materials such as cadmium selenide. a known carcinogen. Further, he said, because it is a physical manufacturing process, there tend to be large discrepancies in the size of dots produced. Using a natural, organic process creates dots that are cheaper and less environmentally damaging to produce, and the results have high levels of purity and are identical in size. “We let nature take its course.”
Inorganic quantum dots already are being used for displays, and previously it was thought that organic dots would only radiate in ultraviolet frequencies. However, by manipulating the chemistry of the dots, Mr. Rosenman has been able to get them to generate colored light. “No one knows these peptides can be caused to vibrate in the visible spectrum,” he said.
Although the technology has a wide range of applications, and Store Dot has protected intellectual property in many areas, it is, for now at least, concentrating on just two: displays and batteries.
In a demonstration, Mr. Rosenman shone a blue light (the backlight in an LCD TV is blue) onto tubes containing different solutions of quantum dots. The tubes lit up in red, green and blue—the constituents of any display. “There is a cost saving of about 10 times compared to other displays,” said Mr. Myersdorf. “The manufacturing process is the same as for making OLEDs.” An OLED is an organic light-emitting diode, commonly found in some smartphones and TVs.
But it isn’t just far-cheaper displays that Store Dot is working on. Mr. Rosenman demonstrated a power cell. By replacing the electrolyte with a solution containing the quantum dots, the same cell had a five-fold increase in charge.
Not only can much more powerful batteries be made (or batteries generating the same power at a greatly reduced size), but quantum-dot-enhanced power cells should not show the same degradation as conventional batteries.
“Because the quantum dots are crystalline, they stay for thousands of charge cycles,” Mr. Rosenman said.
The company is working on a cell for powering a cellphone that would take just seven minutes to charge for daily use.
At the moment the technology is still in the laboratory, but Store Dot is moving to trials and is in talks with cellphone maker Samsung Electronics Co. and others about commercializing the technology.
Subscribe to:
Posts (Atom)




